Our Methods
1. Non-Invasive Transcranial Brain Stimulation
Non-Invasive Transcranial Brain Stimulation (NTBS) can be applied ‘online’ (i.e., during) or ‘offline’ (i.e., before) relative to a respective behavioral measurement or neuroimaging technique. The ‘online’ approach is focusing on the immediate response of the brain to the stimulation. It can be used (a) to quantify local network properties (such as excitability or connectivity) by applying stimuli that are strong enough to evoke a direct output, (b) to interfere with ongoing spontaneous or task-related neuronal activity (and assess the causal relevance of the targeted bran network for a certain cognitive function), or (c) to modulate the level or timing of spontaneous or task-related neuronal activity (to facilitate or inhibit a brain region, gate leaning-related plasticity, or entrain neuronal oscillations). The ‘offline’ approach focusses on the after-effects of the stimulation and is based on synaptic plasticity. Certain NTBS protocols can induce long-term potentiation (LTP)-like or long-term depression (LTD)-like changes in cortical excitability and thereby transiently facilitate or inhibit spontaneous activity a certain brain region before a task or neuroimaging is applied.
1.1 - Transcranial Magnetic Stimulation - TMS
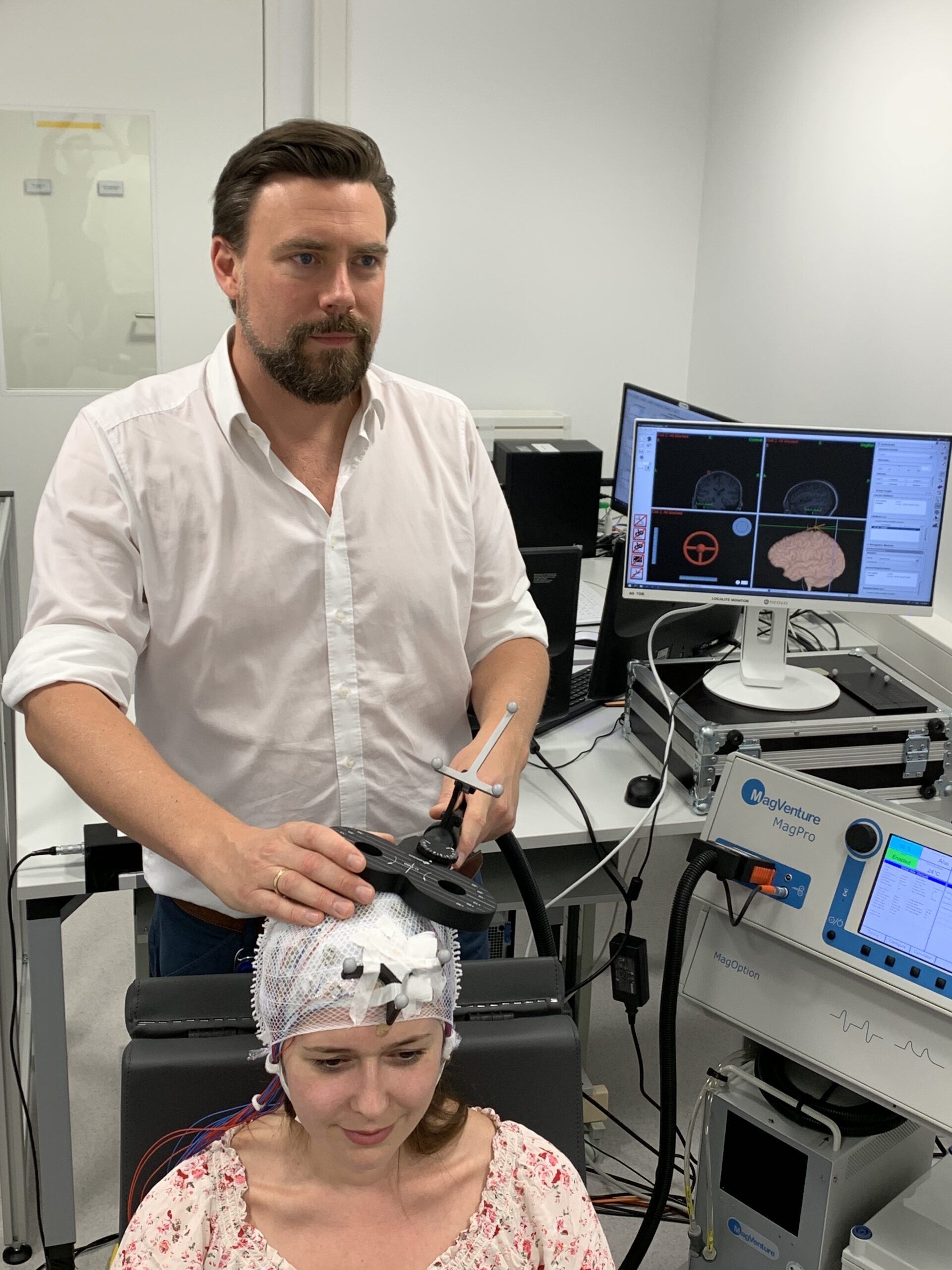
Transcranial Magnetic Stimulation (TMS) is a non-invasive method to stimulate small regions of the brain based on the principle of electromagnetic induction. A magnetic field generator (the TMS coil) is placed on the participant’s head, and for the fraction of a millisecond a strong electric current is discharged through the coil, producing a magnetic field that painlessly reaches into the brain and induces an electric current in the brain tissue, which depolarizes axons and triggers action potentials in cortical neurons. Based on an MR-guided neuronavigation system, the coil can be positioned very precisely to stimulate a circumscribed part of the cortex (and its connected regions). TMS can be applied as single-pulse, paired-pulse, or in bursts for online approaches, and in form of repetitive TMS (rTMS) for offline approaches, and its precise effects depend on the coil location and orientation, as well as the intensity, frequency, and temporal pattern of the delivered magnetic pulses. For example, single-pulse TMS of the primary motor cortex (M1) can evoke twitches in the contralateral muscles, which can be quantified by the amplitude of the motor evoked potential (MEP) in the surface electromyogram (EMG) and indicates corticospinal excitability. Repeated MEP measurements can indicate changes in corticospinal excitability due to spontaneous changes in in brain state or experimental manipulations (e.g., rTMS). Our lab is using TMS mainly in combination with neuroimaging techniques (see below) to measure excitability and connectivity, entrain neuronal oscillations, and induce transient excitability changes.

1.2 - Transcranial electric stimulation - tES
Transcranial electric stimulation (tES) applies very weak currents to the brain via two or more scalp electrodes. Large parts of this current are shunted via the skin, but a small proportion painlessly reaches the brain and slightly de- or hyperpolarizes the membrane potential of neurons, causing a slight increase or decrease in their excitability and thus spontaneous firing rates. Transcranial direct current stimulation (tDCS) applies currents of fixed intensity and polarity, and depending on the exact electrode placement (the montage), as well as the polarity, intensity, and duration of the applied current, the resulting current distribution in the brain results in a net increase or decrease of neuronal excitability in certain brain regions. Our lab is currently using TDCS in combination with TMS to innovate more effective stimulation paradigms.
Transcranial Alternating Current Stimulation (tTACS) is very similar to tDCS but uses an (usually sinusoidal) alternating current at a certain frequency (either with a certain DC offset or by changing polarity with each cycle) to interact with the brain’s potential to spontaneously produce neuronal oscillations. The idea is that the weak oscillating fields in the brain tissue entrain the neurons’ membrane potential and thereby its excitability, eventually time-locking their spontaneous firing to the tACS phase of maximal excitability. When successful, the transcranial entrainment of neuronal oscillations can help to unravel the causal role of neuronal oscillations for certain cognitive functions. Our lab is using tACS, in combination with neuroimaging techniques, to study the function of neuronal oscillations. We are also co-organizing The TACS Challenge, an international multi-centre initiative to test the capability of TACS to phasically modulate behavioural performance.

1.3 - Transcranial ultrasound stimulation - TUS
Transcranial ultrasound stimulation (TUS) is a newly emerging technique for non-invasive neuromodulation in humans, using ultrasonic waves instead of electric fields to interact with neural targets in the brain. Special ultrasound transducers, placed on the participants head, transmit a beam of low-intensity ultrasonic waves through the intact human skull, which can be steered precisely and focused on a few cubic millimetres of brain tissue at several centimetre distance. TUS thereby allows for the first time to non-invasively, reversibly, and exclusively target with unprecedented precision also very small subcortical brain structures, without co-stimulating more superficial cortical regions (which is impossible with TMS and tES). TUS comes with (at least) two key innovations that will likely revolutionize non-invasive neurostimulation in both fundamental research and psychiatry within the next decade: Firstly, TUS allows the direct modulation of local neuronal activity (presumably via the interaction with mechanosensitive ion channels and possibly with the neurons’ membrane itself), with specific protocols being able to induce also plasticity-related after-effects. Secondly, when combined with the injection of microbubbles into the blood stream, TUS is able to transiently and locally open the blood-brain barrier (BBB), thereby enabling the focal uptake of pharmaceutical (or other active) compounds otherwise unable to pass the BBB, providing a mechanism for targeted drug delivery. Current developments in TUS transducer technology will soon allow to flexibly control the focus and shape of the ultrasound beam, enabling multi-focal and moving stimulation patterns adjusted to individual neuroanatomy and specific brain functions. Importantly, the above described applications use low-intensity focused ultrasound (LIFU) only, and the device (NeuroFUS, Brainbox Ltd, UK) and protocols we use do not produce relevant temperature increases in the brain tissue. LIFU for neuromodulation or BBB opening has thus to be distinguished from the high-intensity focused ultrasound (HIFU) protocols produced by neurosurgical devices, which are explicitly used for the non-invasive thermal ablation of brain tissue, e.g., for the treatment of tremor in Parkinson’s disease.

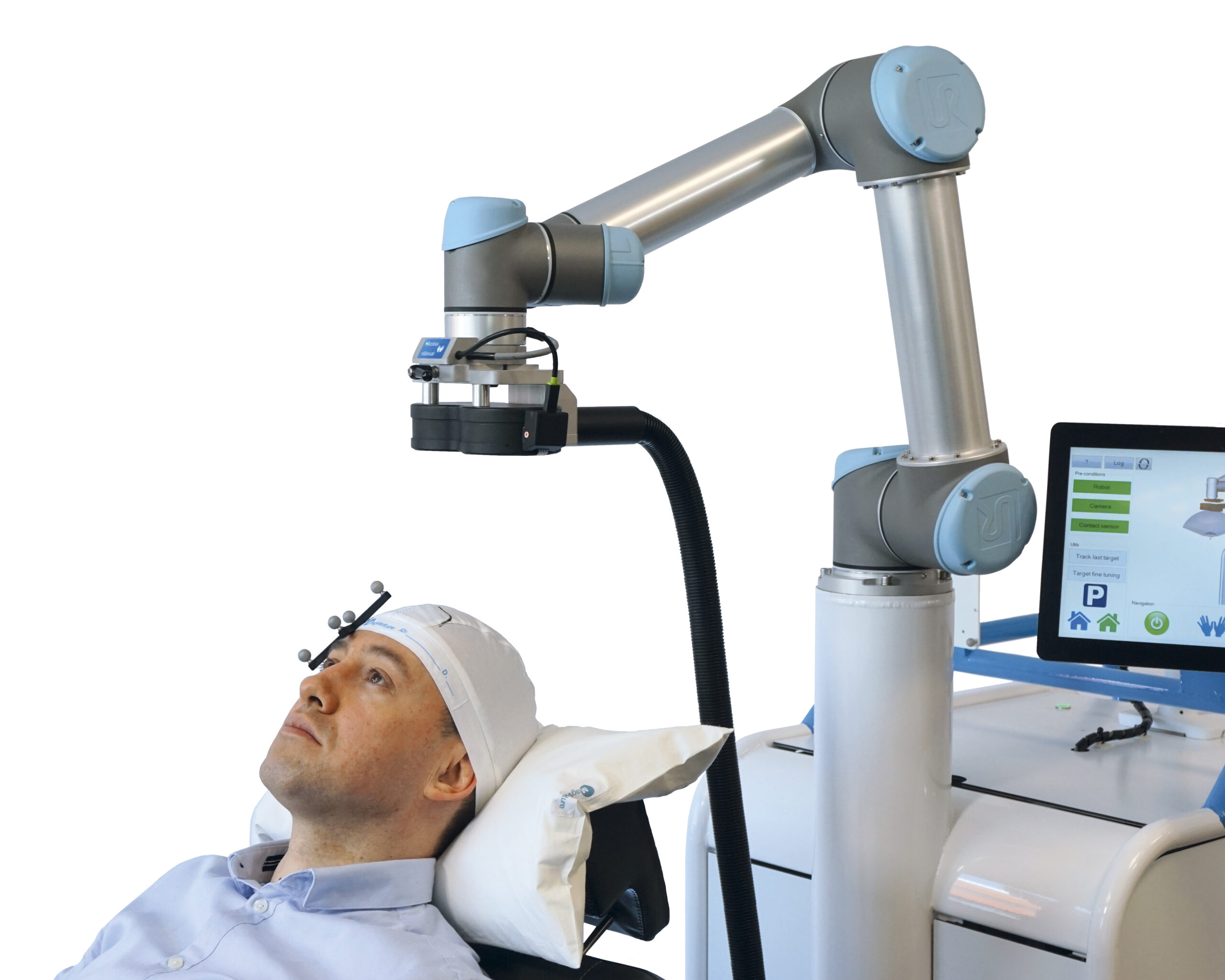
1.4 - Robotized Neuronavigation
Frameless stereotactic neuronavigation allows to co-register the participant’s head to their structural MR image with the help of a stereo infrared camera and so called trackers (unique geometries made of three infrared-reflecting spheres) being attached the head as well as to a pointer with which multiple strategic points on the surface of the participants head are sampled and automatically aligned with the rendered surface of the structural MRI. Additional trackers on the TMS coil or TUS transducer then allow to navigate these relative to the participants brain with millimetre precision and to bring them into the desired position for stimulating the anatomical target structure. While this MR-based neuronavigation can be conducted manually, robot-assisted navigation has become feasible and will likely become the state-of-the-art in the near future. Robotized neuronavigation allows to automatically bring the TMS coil or TUS transducer into the desired position with the help of a robotic arm, to compensate participant’s head movement in real-time, and to target multiple brain regions in subsequent trials, allowing effective mapping of entire target regions or the experimental randomization of target conditions. If integrated with a powerful experimental control software (e.g., our BEST toolbox in section 4.2), robotized neuronavigation also allows to establish automated motor hotspot searches in a closed-loop setup.
2. Multimodal Neuroimaging
2.1 - Electroencephalography - EEG
Electroencephalography (EEG) is the most commonly used method to non-invasively monitor neuronal activity in the brain via surface electrodes attached to the scalp (up to 64 or 128 in an EEG-cap), measuring and amplifying the voltage difference between different electrode sites. The weak EEG potential at the scalp (in microvolts) depends on the summation of extracellular synchronized postsynaptic currents of large neuron populations, producing a polarization of ions in the brain tissue outside the neurons (the local field potential, LFP), Since the EEG signal measured at each electrode reflects a mixture of numerous active sources in the brain, source localization methods are required to make inferences about the local origin of specific EEG phenomena. EEG can be analyzed with respect to the frequency, power, phase, or spatial coherency of spontaneously occurring or experimentally induced oscillations, or the amplitude and latency of certain event-related (evoked) potentials. Our lab uses EEG to study neuronal oscillations during wakefulness and sleep, and to quantify the cortical response to transcranial brain stimulation.
2.2 - Polysomnography - PSG
Polysomnography (PSG) refers to the recording of sleep based on the concurrent measurements of brain waves (electroencephalography, EEG), muscle tone (electromyography, EMG), and eye movements (electrooculography, EOG). Based on these measurements, different sleep stages can be scored: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep as well as sub-stages (N1, N2, N3 as well as phasic and tonic REM sleep). Out lab uses PSG as a standard method to score sleep stages before starting more detailed analyses of certain oscillatory events, such as NREM slow oscillations (< 1Hz) and sleep spindles (~10-16 Hz), or REM delta (~2-4 Hz), theta (~4-8 Hz) and beta (~17-30 Hz).
2.3 - Magnetoencephalography - MEG
Magnetoencephalography (MEG) is an electrophysiological method that uses superconducting quantum interference devices (SQUIDs), cooled with liquid helium, to measure the tiny magnetic field outside the skull that are produced by the summation of the synchronized magnetic fields resulting from the flow of intracellular postsynaptic potentials along the dendritic tree of pyramidal cells. These tiny fields in the range of femtotesla can only be measured in electromagnetically shielded recording chambers. MEG is more sensitive to tangential sources in the brain, whereas EEG mainly picks up radial sources. MEG is also less susceptible to electric volume conduction effects than EEG (but only local field spread) and superior with respect to its spatial resolution, but less flexible in its application compared to EEG. Importantly (for us), it can be combined concurrently with TDCS/TACS, but not with TMS. Our lab uses MEG to study neuronal oscillations at high spatial precision and the brains responses to oscillatory sensory or transcranial current stimulation.
Example Papers
Zhigalov A*, Herring J*, Herpers J, Bergmann TO, Jensen O. (2019). Probing cortical excitability using rapid frequency tagging. NeuroImage, doi: 10.1016/j.neuroimage.2019.03.056. [*contributed equally]
Herring JD, Marshall TR, Esterer S, Jensen O, Bergmann TO (2019). Low-frequency alternating current stimulation rhythmically suppresses gamma-band oscillations and impairs perceptual performance.NeuroImage, doi: 10.1016/j.neuroimage.2018.09.047
Marshall TR, O’Shea J, Jensen O, Bergmann TO (2015). Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipito-parietal cortex. Journal of Neuroscience, 35(4): 1638-1647.
2.4 - (Functional) Magnetic Resonance Imaging – fMRI
Magnetic resonance imaging (MRI) is a non-invasive neuroimaging technique using magnetic fields and radio frequency pulses to acquire images of the human brain with high spatial and decent temporal resolution. Some MR sequences are optimized to provide high-resolution structural information about the grey matter (T1- or T2-weigthed) and white matter (diffusion weighted imaging, DWI) of the brain, whereas other sequences (T2*-weighted) are sensitive to changes in blood flow and blood oxygenation, i.e., the blood oxygenation level dependent (BOLD) signal. Using the BOLD-signal, functional MRI (fMRI) provides an indirect marker of neuronal activation in a time-resolved fashion, since changes in neuronal activity are accompanied by local BOLD-signal changes (a process known as neurovascular coupling). fMRI can be used to study event-related changes in neuronal activity as well as functional connectivity networks during resting-state. Our lab uses fMRI mainly to functionally localize stimulation targets from task- or resting-state fMRI, in combination with transcranial brain stimulation to evaluate it online and offline effects on brain activity, or in combination with EEG to study the brain networks associated with certain neuronal oscillations.
2.5 - Concurrent EEG-fMRI
The concurrent recording of EEG and fMRI allows to co-register the complementing information from both techniques, taking advantage of the high temporal resolution of EEG and the high spatial resolution of fMRI. Given the large magnetic fields and radiofrequency pulses in the MR-environment and the artifacts caused in the EEG due to the magnetic gradients of fMRI sequences and heart-beat related head/electrode movements in the static magnetic field of the MRI, special MR-compatible EEG equipment, specific recording procedures, and sophisticated EEG-artifact correction methods are required to gain high quality EEG-fMRI data. However, their combination is worthwhile when EEG and fMRI data from the same trials or spontaneous events needs to be related. Our group employs concurrent EEG-fMRI to study the brain networks and neuronal activations in subcortical brain structures that are associated with certain oscillatory events in wakefulness and during sleep.
3. Combining Transcranial Brain Stimulation with Neuroimaging
Transcranial Brain Stimulation and Neuroimaging techniques can be combined for different purposes. Neuroimaging (fMRI, EEG, MEG, etc.) can be utilized in a consecutive or concurrent fashion to individualize and optimize the application of transcranial brain stimulation techniques by refining target selection and stimulation parameters such as TMS coil position/orientation, tES montage, stimulation intensity, or frequency. In turn Neuroimaging can also be used as a read-out to assess the immediate (online) neuronal response to the stimulation via concurrent recordings, or the after-effects (offline) based on stimulation-induced changes in synaptic efficacy and cortical excitability via consecutive recordings. Our lab uses MRI, fMRI, and EEG to personalize subsequent brain stimulation procedures, but has also a particular focus on the concurrent application of TMS-EEG, TMS-fMRI, and tES-MEG, as well as brain state-dependent stimulation.
3.1 - Concurrent TMS-EEG
On the one hand, concurrent TMS-EEG can either be used to measure the direct brain response to TMS, the TMS-evoked EEG potential (TEP) or TMS-induced oscillations (TIO), in regions outside the primary motor cortex (M1), where motor-evoked potentials (MEP) cannot be assessed. TEPs can be utilized to index cortical excitability, effective connectivity, and the general complexity and spectral composition of a brain response. TMS causes a number of complex artifacts in the EEG, resulting from magneto-electric induction, electrode/electrolyte depolarization, vibration, cranial muscle activation, etc. that need to be attenuated during the recording and removed during pre-processing before EEG analysis (respective pipelines exist for FieldTrip and EEGlab). However, TMS is inevitably associated with sensory-co-stimulation effects from auditory (the TMS ‘click’ sound) and somatosensory input (excitation of peripheral and cranial nerves, and cranial muscle twitches) resulting in peripherally evoked potentials (PEPs) that need to be attenuated and actively controlled for. On the other hand, concurrent EEG-TMS can be used to inform the timing (and other stimulation parameters) for real-time EEG-triggered TMS (brain state-dependent brain stimulation, see below), e.g. to study neuronal oscillations.
3.2 - Concurrent TMS-fMRI
Concurrent TMS-fMRI allows to use the TMS-evoked BOLD-signal to assess the neuronal response of the brain to transcranial cortical stimulation, revealing both the local response at the stimulation site itself and the network response in remote connected regions, including deep subcortical structures. Beside the assessment of brain networks and effective connectivity, TMS-fMRI can provide ‘proof of target engagement’, that is empirical evidence for having effectively stimulated the targeted brain region. This is particularly relevant when the actual target site is a deep structure that cannot be directly accessed by TMS, and therefore requiring the transsynaptic stimulation via projections from a more superficial cortical entry point. Measuring the direct BOLD-response to stimulation also allows to determine the optimal stimulation parameters (coil orientation, intensity, frequency, etc.) for a certain target region, going beyond the mere referencing to the individual resting motor threshold (RMT) even for non-motor regions at different scalp-cortex distance and orientation. Our lab is currently establishing TMS-fMRI to optimize stimulation parameters for both superficial and deep target structures, with the aim to demonstrably stimulate memory- and emotion-related deep brain structures and to investigate their role in fear memory consolidation and emotional memory updating in the context of resilience.
3.3 - Concurrent tES-MEG
The application of TDCS or TACS with concurrent MEG recordings allows to study how neuronal oscillations or evoked fields are modulated by the presence of weak electric fields transcranially applied to the brain. Advantages over concurrent tES-EEG are that brain signals can more easily be recorded from areas directly underlying the stimulation electrodes (whereas no EEG electrodes can be placed overlapping with stimulation electrodes), and that larger current intensities are possible without saturation of the recording system. The disadvantage is that for MEG the head with the tES-electrodes (and thus the strong dipole of the stimulation circuit) can move relative to the sensors, causing non-linear heart-beat and respiration-related artifacts. Our group is using concurrent tES-MEG recordings to study the modulation and entrainment of neuronal oscillations.
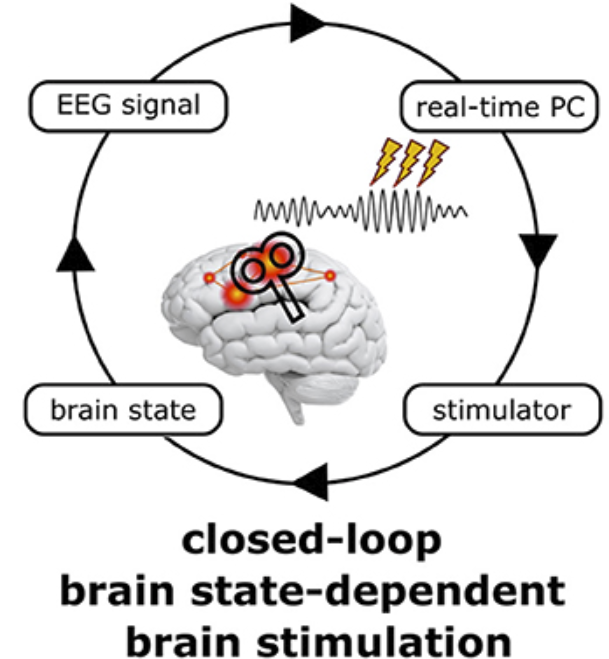
4. Brain-State Dependent Brain Stimulation
Brain State-Dependent Brain Stimulation (BSDBS) is a novel approach, relying on the simultaneous application of neuroimaging and neurostimulation techniques, such as concurrent EEG-TMS. For the last 30 years, non-invasive transcranial brain stimulation (NTBS) approaches, using transcranial magnetic stimulation (TMS) and transcranial electric stimulation (tES), have treated the brain as a black box, ignoring its internal state at the time of stimulation. While inter-individual variability is long known to undermine the replicability of NtBS effects, intra-individual variability across and within sessions has only recently gained attention. NTBS effects are state-dependent on a time scale of minutes to hours, depending on the immediate history of neural activity and synaptic plasticity. However, brain states also change on the time scale of seconds to milliseconds, as neurons are heavily influenced by the temporospatial dynamics of spontaneous network activity, governed by rhythmic fluctuations in neural excitability and under the control of ascending neuromodulatory systems and thalamo- and cortico-cortical projections. Frequency, amplitude, and phase of neuronal oscillations constitute transient local, network, or even global brain states that not only determine the fate of incoming sensory stimuli, but also affect both the immediate (‘online’) neuronal response to NTBS and the subsequent after-effects (‘offline’) resulting from NTBS-induced synaptic changes. Taking the current oscillatory brain state into account when applying transcranial brain stimulation thus not only allows to study brain states and the function of neuronal oscillations. It may also help to control this factor of variability and obtain more consistent results. Technical advances now allow to assess ongoing multi-channel EEG data in real-time and modify stimulation parameters on the fly to apply brain state-dependent brain stimulation (BSDBS). Our group mainly uses BSDBS to target specific oscillatory events, or oscillations at particular phases and amplitudes to study their underlying neurophysiology and their function in information processing and plasticity in the sleeping and awake human brain. We are also actively developing our technical setup to further improve the precision and flexibility of real-time EEG-triggered TMS, to eventually fully closed-loop BSDBS.
Example Papers
Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, Siebner HR (2012).EEG-Guided Transcranial Magnetic Stimulation Reveals Rapid Shifts in Motor Cortical Excitability during the Human Sleep Slow Oscillation. Journal of Neuroscience, 32:243-253.
5. Software Developments
5.1 - MAGIC Toolbox
The combination of transcranial brain stimulation with neuroimaging techniques requires technically sophisticated and digitally controlled stimulation protocols, allowing to flexibly and remotely control the TMS devices, changing stimulation parameters (intensity, frequency, inter-stimulus interval, direction of current flow, pulse form, etc.) on the fly. Together with our collaborators (Nigel Rogasch et. al., Australia ) we developed the MAGIC (MAGnetic stimulator Interface Controller) toolbox, an open-source MATLAB toolbox for external control of transcranial magnetic stimulation devices (currently MagStim, MagVenture, and DuoMAG machines).
GitHub Repository: https://github.com/nigelrogasch/MAGIC/wiki
5.2 - BEST Toolbox
To further reduce human error and variability, we aim to automate and standardize experimental procedures for the application of brain stimulation protocols and the acquisition of the respective brain responses (in EMG, EEG, and fMRI) as far as possible. Together with put collaborators (Christoph Zrenner, Tübingen) we currently develop a software toolbox for planning, controlling, and analyzing TMS-EEG/EMG data in real-time. The BEST (Brain Electrophysiological recording and STimulation) toolbox is an open-source MATLAB toolbox for closed-loop brain state-dependent brain stimulation (www.best-toolbox.org). The BEST toolbox have also integrated bi-directional interaction with (robotized) neuronavigation systems, and the remote control of the NeuroFUS device for TUS.
Example Papers
Hassan, U., Pillen, S., Zrenner, C., & Bergmann, T. O. (2021). The Brain Electrophysiological recording & STimulation (BEST) toolbox. Brain Stimulation. doi:10.1016/j.brs.2021.11.017

